At Kerlo Research, we understand the challenges inherent in medical monitoring, including protocols lacking precise guidance, the absence of real-time data trackers to identify issues promptly, and the lack of a comprehensive Medical Monitoring Plan. We have created a comprehensive suite of services to combat these issues and streamline your research activities.
The Medical Monitor Role
Acting as the unwavering advocate for subject safety and well-being, the medical monitor assumes responsibility for:
- Individual patient safety monitoring and may conduct medical assessments of the clinical study protocol.
- They offer crucial input into decisions concerning study design, treatment regimens, comparator selection, medical data interpretation, and contribute significantly to data analysis and report generation.
Common Pain Points
- The CRO demonstrates insufficient comprehension of the protocol.
- The protocol may be deficient in providing specific guidance.
- eCRF templates and instructions are possibly ambiguous.
- Absence of an effective Medical Monitoring Plan.
- Data is not consistently reviewed.
- Listings/patient profiles are not tailored to meet data review objectives.
- Real-time data issue identification hindered by the absence of data trackers.
- Vendors lack clarity regarding their responsibilities.
Our Comprehensive Approach
- Medical involvement in the development of study documents, protocols, training materials, and ensuring alignment and guidance.
- Evaluating subject eligibility.
- Providing guidance on medical matters and reviewing subject safety data.
- Assessing and reviewing protocol deviations and implementing mitigation plans, protocol amendments, and newsletters.
- Reviewing eCRF templates, eCRF instructions, and the Medical Monitor Plan.
- Contributing to safety review committees.
- Regular cross functional team meetings.
- Open communication with study sites.
- Track query responses assuring timely resolution.
- Understanding of services provided by the CRO/Vendors.
- Tailoring listings and patient profiles to align with data review objectives.
- Standardizing the data review process.
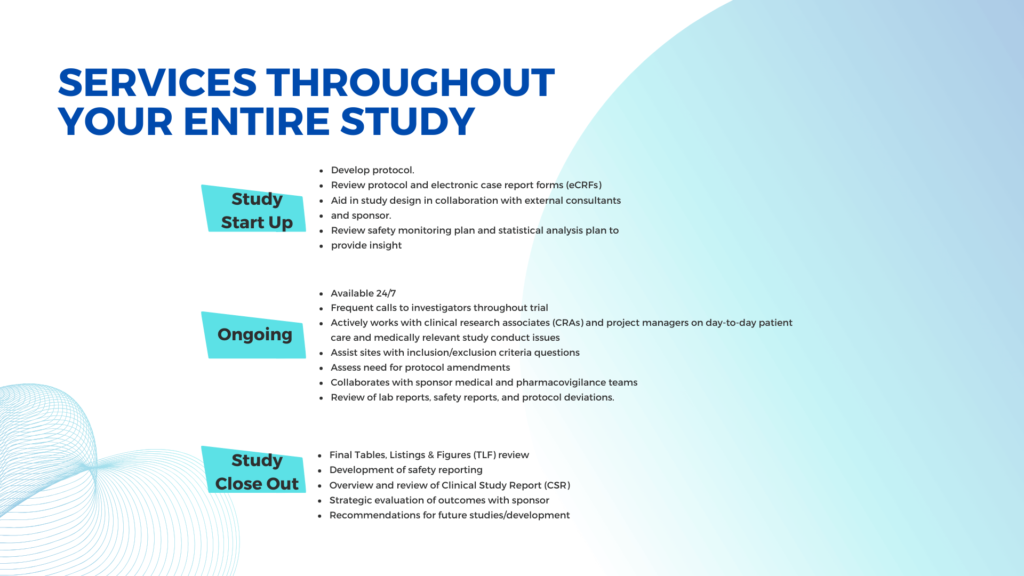
Following our commitment to addressing these challenges, we offer comprehensive services including Start-up, Ongoing Trial, and Close-out services to ensure seamless execution and successful outcomes for every clinical trial project.
Our cutting-edge medical monitoring services are powered by a state-of-the-art cloud-based software system developed by our esteemed IT team. This innovative solution is designed to streamline the data analysis process, significantly reducing both time and resources required. With our advanced technology, we can ensure quicker turnaround times, delivering results to sponsors promptly and efficiently. Welcome to a new era of medical monitoring excellence, where precision meets speed.
Don’t let inadequate medical monitoring compromise your clinical trial’s success. Reach out to us todayfor a comprehensive consultation and discover how our experienced team can tailor our services to meet your specific needs, ensuring the highest standards of safety and data integrity throughout your trial journey.

